Bioness FES Prescription at Orthotics Plus
Functional Electrical Stimulation (FES) systems are devices used to provide electrical stimulation to a muscle to generate movement.
Unlike TENS machines and therapeutic devices that are used statically, FES systems are utilised in the real world while a patient is walking to add functional movement and increase mobility.
The Bioness FES system is a high-quality brand of FES system that Orthotics Plus prescribes to suitable patients.
The Bioness is a cuff that is placed below the knee, and when a wearer takes a step, the device provides a low-level stimulation to the tibialis anterior and peroneal muscle groups, generating lift (dorsiflexion) at the ankle. This system can also be used on the quadriceps muscles, although it’s not as common as its application below the knee.

Clinical Application of Bioness FES in Neuro-Rehabilitation
The Bioness FES device has two clinical applications:
- Orthotic Effect: The device restores movement and improves the biomechanics of individuals who have difficulty lifting their foot or extending their knee. This leads to improved walking ability, increased safety, and greater overall mobility.
- Therapeutic Effect: The device stimulates the targeted muscle and provides sensory input to the brain. This promotes muscle strengthening and neuroplasticity. As a result, individuals may experience improved rehabilitation outcomes and better walking ability even when not wearing the device. The device does not ‘do the work’ for the individual but rather assists the body to ‘do the work’, leading to constant rehabilitation with each step taken.

Key Differences Between the Bioness & Other FES Systems on the Market
In comparison to other systems, the Bioness FES is distinguished due to its electrode placement flexibility, tunability and responsiveness. The device offers a variety of electrode types, allowing for optimal placement and stimulation of the correct muscle groups. Other FES devices may offer fewer options in terms of electrode placement.
The Bioness FES device also has independent channel tuning, which is not available on all FES devices which allows the clinician to program each electrode individually.
Additionally, it features an accelerometer, tilt sensor, and gyroscope to sense the unit’s position in space, providing greater accuracy when detecting movement. This enables the device to recognise sidestepping, walking backwards, and other unique movements and stimulate the leg appropriately.

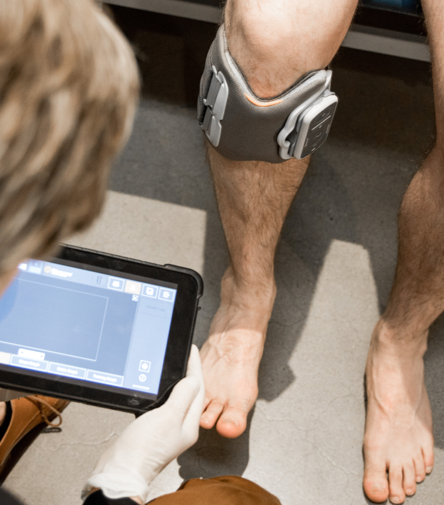
Contraindications to the Bioness FES
The Bioness FES device has certain contraindications that must be considered before use.
Upper motor neuron lesions are required for the device to work properly as the pathway from the brain spinal cord to the muscle group needs to be intact or mostly intact.
It may be contraindicated by:
- Individuals with complete spinal cord injuries or those who have severed nerves between relevant muscle groups to the spine
Spasticity can also be a limitation. However, spasticity can also respond well to the device. - Skin irritation at the site of electrical stimulation and discomfort from the stimulation itself may also occur, but these can be managed. It is important to ensure that the patient can tolerate the stimulation, as some individuals may have a low tolerance to the device, which can limit its effectiveness. Patients can increase their tolerance over time, but it may take some effort to push through any initial discomfort.
Pregnant individuals or those with pacemakers can use the device with approval from relevant professionals.
Overall, the Bioness FES device is safe and effective for many individuals, but careful consideration of contraindications and limitations is necessary.

Feedback From Bioness FES Wearers
We have had dozens of excellent results with various FES systems and in particular the Bioness. For example, one patient had a remarkable improvement using the Bioness FES device, going from being only able to walk 100 meters with a walking stick, to walking seven kilometres unaided within a few months.
For many patients, the improvement is more subtle but still significant. They report feeling like their limb is lighter and less fatigued at the end of the day, which lowers the risk of falling.
Additionally, patients appreciate the ability to wear any shoes they want, and the device’s cosmetic appearance is appealing to people.
Many of our FES patients are current or previous AFO users and they report not having to wear an AFO in their shoe to be more comfortable and freeing with shoe selection.
The device also enables patients to walk barefoot outside and walk around their homes without shoes, which was not possible with their previous assistive devices.

Funding and Comparisons with Other Assistive Technology
Typically, AFOs are a consideration and assessed for suitability. However, for patients whose objectives include walking barefoot or barefoot-like, and those who want to optimise their potential for rehabilitation, FES systems are a viable option.
FES systems, in our opinion, should be a primary treatment for stroke patients due to their neuroplastic and therapeutic effects.
The question is whether the Bioness device is the most suitable or if a less expensive FES system would produce appropriate results.
The answer is that sometimes the lower-cost options are preferred by patients due to their simplicity or appearance and also they may fit within a patient’s personal or NDIS budget. However, for patients regularly performing complex movements like sidestepping or walking backwards (for example, patients in the kitchen regularly or those with small children) the Bioness system is the favoured option.

Ready to Enquire About a Bioness FES?
Orthotics Plus is an NDIS-registered provider proudly serving the Melbourne community.
- We stock many types of low cost, low risk Assistive Technology
- We stock many types of high-cost Assistive Technology
- We promote choice and control and enable in-clinic trials as much as feasible
- We provide equipment prescriptions and help people access appropriate healthcare solutions under the NDIS
To contact us, please visit our clinic locations page. We look forward to your enquiry.

ABI FAQ
As part of our process, we provide instructions to all individuals regarding the usage and maintenance of their orthotic devices.
We also take into consideration the convenience and ease of use for both the patient and their caregivers. As an example, we assess and recommend appropriate footwear options that are caregiver-friendly and easily removable. This may include footwear with Velcro straps instead of laces, ensuring simplified dressing.
We take the time to demonstrate and explain different techniques for correctly fitting devices. It is crucial to effectively communicate this information to both the patient and their caregivers for proper utilisation and maintenance.